Quality
Providing Quality
Biotexus has a strong, never ending commitment to quality.It is one of the crucial traits on which we prioritize the most and will not compromise.
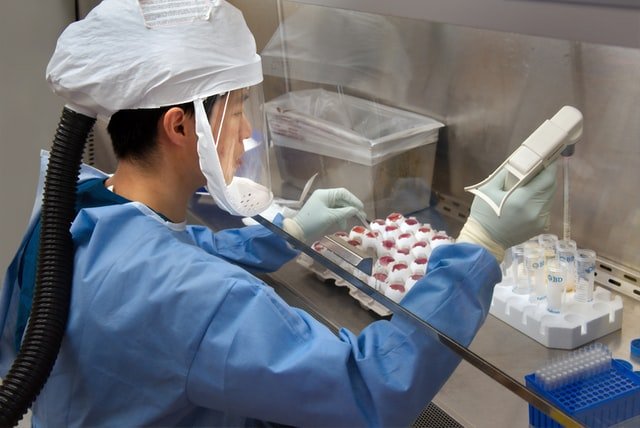
We’re dedicated to attaining zero defects and employ stringent quality controls to ensure that every product released meets all applicable quality and regulatory standards. To guarantee that the finished goods are uniform, safe, effective, and predictable, each product’s manufacturing process is subjected to severe quality standards. Our commitment to maintaining the highest quality standards has earned us a reputation as a leader in the pharmaceutical industry. We believe that maintaining high quality standards in everything we do is the key to building a responsible brand’s foundation. Quality is ingrained in all aspect of our business, including procurement, production, delivery, and product disposal. We focus on improving our Quality Management Systems to meet and surpass the current standards of regulatory agencies such as the CDSCO, US FDA, MHRA, TGA, MCC, WHO, and others.

Transforming Oncology & Nephrology with innovative medicines, prioritizing patients, quality, affordability, and forging enduring connections through excellence.
Our Quality Control (QC) team designs and implements robust practises to assure highest quality throughout the manufacturing process. Safe destruction of defective and expired items, as well as strict pharmacovigilance is maintained post manufacturing. The Quality by Design (QBD) and Development Quality Assurance (DQA) teams collaborate closely with the R&D department to ensure highest quality during clinical trials, technology transfer, and the development of new compounds.






Our Vision towards Quality //////
At Biotexus, we strive to enhance our Quality Management System (QMS) and all of its components on a continuous basis. Through ongoing growth, training, and empowerment of our employees, we are establishing and maintaining a high-quality culture. We feel that it is everyone’s obligation to produce a safe, high-quality product.
Assurance of High Quality
We recognise that making adequate and timely investments in improving our quality management standards will pay off in the long run by reducing the cost of failure, increasing stakeholder confidence, and ensuring legal compliance. We offer cutting-edge manufacturing facilities that follow cGMP guidelines in accordance with national and international regulations. Our facilities maintain quality by upgrading equipment on a regular basis, adopting technological breakthroughs, and following industry best practises.
Assurance of High Quality
We recognise that making adequate and timely investments in improving our quality management standards will pay off in the long run by reducing the cost of failure, increasing stakeholder confidence, and ensuring legal compliance. We offer cutting-edge manufacturing facilities that follow cGMP guidelines in accordance with national and international regulations. Our facilities maintain quality by upgrading equipment on a regular basis, adopting technological breakthroughs, and following industry best practises.
/////// Quality System Management
The Global Quality Manual provides all of the QMS’s quality system features that apply to all of our business lines. We employ best practises to ensure that patients receive consistent, high-quality products. At Biotexus, we have a single quality standard that we adhere to all across the India. We ensure that it is implemented across several functions, including R&D, quality, and technical operations.

Strategy for self learned lesson /////
As a consequence of our extensive Quality Control systems, we have effectively faced problems such as increased regulatory demands, demanding compliance requirements, and stringent quality standards. The Company operates on a business model with a forward-looking approach that anticipates regulatory changes. Our “Lessons Learned” technique is ingrained in our quality culture. The process entails identifying gaps, analysing them, mitigating risks, and taking corrective action. This method not only tackles the difficulties, but also leads to actions to prevent repeat occurrences, as well as identifying what went well and how similar processes could benefit from this information.